Synthesis of 4-Chloro-2,5-Dimethoxyphenethylamine (2C-C)[ Back to the Chemistry Archive ] 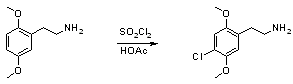
2,5-Dimethoxyphenylethylamine (1.81g, 10 mmol) was dissolved in 20ml glacial acetic acid in a 50ml beaker equipped with magnetic stirring and a thermometer, and placed in an ice-bath. Sulfuryl chloride (2.0g, 1.20ml, 15 mmol) was added dropwise1 to the solution over 30 minutes, the whole time keeping the temperature of the solution between 15-20°C2. When the addition was finished, the ice-bath was removed, and the solution allowed to warm up to room temperature (25°C) and stirred for another 90 minutes, the temperature being monitored occasionally so that it didn't exceed 30°C. A too high reaction temperature causes partial dichlorination of the substrate, a temperature of 40°C during the addition invariably causes side reactions. When the mixture had reacted for a total of 2 hours, 20ml of dry diethyl ether was added, and the solution stirred at room temperature for another 30 minutes, followed by filtration of the precipitated 4-Chloro-2,5-Dimethoxyphenethylamine Hydrochloride and washing with 15ml of dry ether, and air drying to give 0.54g of a white powder. The mother liquor was diluted with an equal amount of dry ether and allowed to stand overnight in the fridge, giving a second crop of 0.74g 2C-C.HCl. The combined crops weighed 1.28g (51% yield) which were and dissolved in a boiling mixture of 50ml acetonitrile and 5ml methanol, which after cooling somewhat was diluted with 30ml dry ether and placed in the fridge to fully crystallize to give 0.67g (27% yield) of pure 4-Chloro-2,5-Dimethoxyphenethylamine (2C-C) Hydrochloride after filtration, washing with 10ml ether and air drying. The recrystallization was very wasteful, and a different solvent system should have been used. Reference [1] suggests methanol/ether. Notes:
References[1] Tetrahedron Letters 42, 3247-3249 (2001) |