Abstract
A selective preparation of 2,5-dimethoxy-4-nitrobenzaldehyde is proposed in a procedure involving the transformation of the aldehyde group to a diacetoxymethyl group.
Quinone containing macrocycles have attracted considerable attention in recent years1-5 since they offer a basis for the chromogenic or electrochemical detection of a variety of metal cations and model biomimetic systems for metalloenzymes. On the other hand, naturally occuring macrocycles containing the quinonoid system are potent antifungal6 or antitumour7 antibiotics.
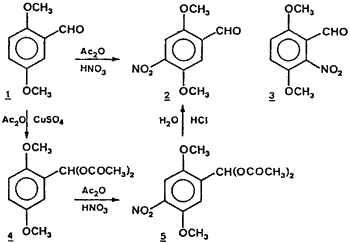
In the course of our studies on cyclic peptides containing the quinone moiety, it was necessary to prepare 2,5-dimethoxy-4-nitrobenzaldehyde2 on a multigram scale. Unfortunately, although 3- and 6-nitro derivatives can be easily obtained in good yields from 5-methoxysalicylaldehyde6 and 2,5-dimethoxybenzaldehyde8 1 respectively, the 4-nitro derivative is only a minor product of the nitration of 2,5-dimethoxybenzaldehyde. Furthermore, its purification involves laborious fractional crystallizations from ethanol. Thus, we sought to modify the orientation of the reaction by the use of different solvents and found that nitration of 1 in acetic anhydride gave the 4-nitro derivative as the major product (91%) and the 6-nitro derivative as the minor product (9%) with an overall yield of 89%. However, when the hydrolysis of the reaction mixture was not complete, we isolated small amounts of 2,5-dimethoxy-4-nitrobenzaldehyde diacetate 5.
Table I:
Nitration of 1 and 4 in AcOH or Ac2O
Run | Starting material | Solvent | Overall Yield | 2 | 3 |
| 1 | 1 | AcOH |
86% |
26% |
74% |
| 2 | 1 | Ac2O |
89% |
91% |
9% |
| 3 | 4 | Ac2O |
90% |
100% |
0% |
| 4 | 1 | Ac2O |
85% |
100% |
0% |
This result leads us to prepare 2,5-dimethoxybenzaldehyde diacetate9 4 and nitrate it. As we expected, 4 afforded the 4-nitro derivative selectively in excellent yield (90%). This selectivity is thought to be due to the bulky diacetoxymethyl group and/or to its electron-donating power (in spite of the reduction of the electron-donating ability of the methyl group by introducing two acetoxy groups). Finally, 2 can be easily obtained in a one-pot procedure without purification of the intermediate compounds.
Experimental
TLC analyses were performed on a 3x10 cm plastic sheet precoated with silica gel 60F254 (Merck), 0.2 mm (Solvent system: ethyl acetate/ hexane 2:3).
2,5-Dimethoxybenzaldehyde diacetate
This compound was prepared as previously described9 in 96% yield, mp 110°C.
Nitration of 2,5-Dimethoxybenzaldehyde diacetate
20 mL of nitric acid (70%, 320 mmol) was added dropwise to a cooled solution of 4 (6.7g, 25 mmol) in acetic anhydride (50 mL) at such a rate as to maintain the temperature between 10-15°C. After being stirred for 1 hour, water (250mL) was added and the solid was filtered off and dried to give a mixture of 5 (70%) and 2 (30%). This mixture was stirred with 1N HCl (50mL) for 2 hours (the reaction was followed by TLC) and extracted with ether (3x50mL). The ethereal layer was dried over Na2SO4 and evaporated to give 2 (4.75g). 5 mp 168-70°C.
Nitration of 2,5-Dimethoxybenzaldehyde
The procedure was the same as for 4. A complex mixture of 4-nitro- and 6-nitro-2,5-dimethoxybenzaldehyde and their respective diacetates was obtained. The acidic hydrolysis gave 2 (91%) and 3 (9%) with an overall yield of 89%. 2,5-Dimethoxy-6-nitrobenzaldehyde diacetate; mp 171-2°C.
One-pot preparation of 2,5-Dimethoxy-4-nitrobenzaldehyde
A solution of 1 (4.15g, 50 mmol) and CuSO4·7 H2O (0.05g) in acetic anhydride (50 mL) was allowed at room temperature for 24 hours. After being cooled (10-15°C) 20 mL of nitric acid (70%, 320 mmol) was added dropwise. The solution was stirred for 1 hour and 1N HCl (250mL) was added. The mixture was stirred for 2 hours and extracted with ether (3x50mL). The ethereal layer was dried over Na2SO4 and evaporated to give 2 (4.5g, 85% yield). The same reaction on 200 mmol of 1 gave an 80% yield.