The reaction of indolylmagnesium iodide with substituted alkyl halides, e.g., the nitriles Cl(CH2)nCN and chloroacetyl diethylamide, to give the corresponding 3-substituted indoles is well established1,2 although very reactive halides, such as methyl iodide and benzyl chloride, occasionally give 1,3-disubstituted indoles3. We report now that indolylmagnesium iodide is alkylated by β-dimethylaminoethyl chloride to afford N,N-dimethyltryptamine4. The method appears to be general and, although the reaction conditions are critical, it provides a convenient direct synthesis of substituted tryptamines.
The indolylmagnesium iodide reagent was prepared in the usual manner2 from an indole and ethylmagnesium iodide in dry anisole at 10°C. The mixture was cooled to -5°C and a solution of 2-molar equivalents of β-dimethylaminoethyl chloride in dry benzene was added. After 8 hours at -5°C the mixture was allowed to warm slowly to room temperature, left overnight, and then added to aqueous ammonium chloride. The product was obtained via extraction into dilute hydrochloric acid and subsequent neutralisation.
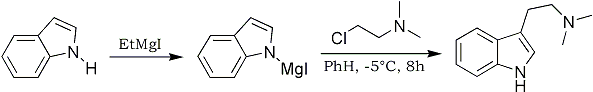
Indole, 2-methylindole and 2-phenylindole afforded respectively, 25, 30 and 15% conversion into the corresponding dimethyltryptamines. These were identified by elemental analysis and comparison of infrared spectra and mp with authentic materials5,6. In each case the crude basic product was subjected to thin layer chromatography and no other substituted indoles were detected.
Alternative conditions were investigated for the reaction with 2-phenylindole. We found that, in anisole, a higher temperature decreased the yield (e.g., only 3% conversion at 40°C) and no alkylated products were obtained from reactions in tetrahydrofuran or in ether, either in the cold or upon heating.
The C-alkylation of indolylmagnesium iodide is particularly interesting in comparison with other metal indole derivatives. We found that 2-phenylindolyl sodium prepared from the indole and sodium hydride, did not react with β-dimethylaminoethyl chloride in anisole at -5°C, but at a higher temperature N-alkylation occurred7 and after 4 hours at 80°C 1-dimethylaminoethyl-2-phenylindole was obtained in 70% conversion. The latter, which has not previously been reported, was recrystallised from light petroleum to give needles having mp 64-66°C (hydrochloride, needles, mp 223-224.5°C). Similar results were obtained in tetrahydrofuran or dimethylformamide and in no case could the C-alkylated product be detected.
The lithium derivative of 2-phenylindole was prepared by exchange with butyl lithium in benzene, anisole was then added and most of the benzene was removed by distillation. The reaction with β-dimethylaminoethyl chloride for 4 hours at 84°C gave the N alkylated product in 23% conversion while at -5°C for 8 hours less than 1% conversion was effected. In neither case was there evidence of C-alkylation.
The contrast between N-alkylation of the indolylsodium and C-alkylation of the indolylmagnesium derivatives is interesting and the reduced nucleophilicity of the indolic nitrogen of the magnesyl compound indicates that the nitrogen is shielded from attack or is stabilised by co-ordination. If indolylmagnesium and indolylsodium are essentially similar ionic species, as has been suggested by Reinecke et al.8, our results imply that, at least in anisole, the indolylmagnesium complex is not dissociated. If the magnesium is associated with the indolic nitrogen then co-ordination of the solvent to magnesium could provide effective shielding and so prevent N-alkylation. Interpretation is complicated by the solvent effect whereby the reaction has been favoured by anisole, the less basic ether.
Further discussion of the mechanism is limited by the possibility that at least two alkylating species may be present. For instance there is good evidence9 to support the view that β-dimethylaminoethyl chloride, though formally an alkyl halide, usually reacts with nucleophiles via the dimethylethylene-immonium ion. The alkylation of indolylmagnesium at -5°C, but not at higher temperatures, certainly implies a competing reaction.