Interest in the biogenesis and pharmacological properties of the ergot alkaloids has led to a number of attempts to convert ergot clavine alkaloids to lysergaldehyde (4, R = H) and lysergic acid (4, R = OH) derivatives but these efforts have been largely unsuccessful1,2. The conversion of elymoclavine (1) to lysergic acid methyl ester (4, R = OCH3) has now been achieved in nearly 30% yield and is the subject of this report.
Bach and Kornfeld3 described the synthesis of 10-methoxy-delta8,9-lysergaldehyde (2) by the manganese dioxide oxidation of the 10-methoxy derivative of elymoclavine obtained by reduction of the methoxy ester 3 (R = OCH3)4. We later prepared 2 in 55% yield by the direct oxidation of elymoclavine (1) with manganese dioxide in methanol5.
The cyanide-catalyzed oxidation6 of the aldehyde 2 with manganese dioxide in methanol provided the methoxy ester 3 (R = OCH3), mp 184-186°C, in 65% yield7. Reduction of 3 (R = OCH3) with zinc and acetic acid3 gave nearly 80% yield of lysergic acid methyl ester (4, R = OCH3), mp 164-166°C. The identities of 3 and 4 were verified by comparison of their TLC behavior, UV, IR, NMR, and mass spectra with those of authentic reference compounds.
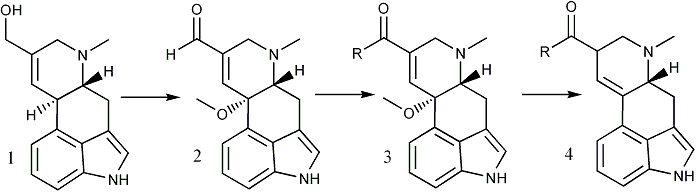
Cory, et al.6 found that the cyanide-catalyzed oxidation of aldehydes in methanol could be directed to give either the methyl ester (with active MnO2) or carboxylic acid (with AgO). However, our attempts to prepare lysergic acid (4, R = OH) via the argentic oxide oxidation of 2 have thus far been unsuccessful. Based upon the work of Gilman9 it was also anticipated that lysergic acid amides could be prepared by substituting the appropriate amine for methanol in the oxidation of 2. By this means we have prepared the diethylamides 3 and 4 (R = NEt2) and therefore expect that this method will prove to be general for the preparation of lysergic acid amides. Thus, lysergic acid derivatives have been prepared from ergot clavine alkaloids in reasonable yield for the first time.