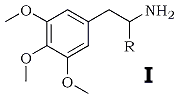
The widely studied pharmacology of mescaline (I; R = H) and the recent descriptions of the psychotomimetic effects dl-trimethoxyamphetamine1,2 (I; R = CH3) have prompted the synthesis of higher homologs (I; R = C2H5 through C7H15). All of the amines were prepared by the LiAlH4 reduction3 of the corresponding nitrostyrenes, which were in turn prepared (with the exception of the nitropropane isomer mentioned below) by the ammonia-catalyzed condensation of the appropriate nitroalkane with 3,4,5-trimethoxybenzaldehyde, in acetic acid.
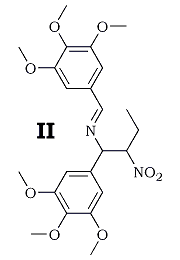
With nitropropane in acetic acid, the solid product was identified as 3,4,5-trimethoxybenzonitrile4, and substitution of isopropyl alcohol for the acidic solvent led to unexpected formation of the Schiff base II, mp 151.5-152°C from methanol, the structure of which was established by analysis and its facile acid hydrolysis to trimethoxybenzaldehyde and, after neutralization, 1-(3,4,5-trimethoxylphenyl)-2-nitrobutylamine. Replacement of the ammonia catalyst with a secondary amine precluded the formation of II, and led to a proper nitrostyrene. The properties and yields of the substances studied appear in the Table.
R | Nitrostyrenesa |
Phenethylamines | |||
mp |
Yield |
mp (picrate) |
mp (HCl) |
Yield |
|
-H |
120-121°Cb |
64% | 214-216°C |
180-181°C |
86%c |
-CH3 |
93-94°Cd |
50% | 171-173°C |
208-209°Ce |
62% |
-CH2CH3 |
72-73°C |
29% | 177-181°C |
192-193°C |
52% |
-(CH2)2CH3 |
82.5-83.5°C |
32% | 182-184°C |
214-218°C |
65% |
-(CH2)3CH3 |
73-74°C |
34% | 168-170°C |
182-184°C |
45% |
-(CH2)4CH3 |
54-55°C |
24% | 162-163°C |
155-158°C |
50% |
-(CH2)5CH3 |
51-52°C |
21% | 149-151°C |
132-134°C |
53% |
-(CH2)6CH3 |
43-44°C |
19% | 148-149°C |
112-116°C |
19% |
-(CH2)8CH3 |
46-47°C |
16% |
- |
- |
- |
- All listed compounds were yellow crystalline solids,
recrystallized from methanol. - Literature values are reported from 118 to 121°C.
It was found that if the molten material was held at this
temperature for a short period, resolidification occurred
yielding a new crystal form with a melting point 128-129°C. - Literature value included for comparison, see 3.
- Literature value 95°C, see
P. Hey, Quart. J. Pharm. Pharmacol. 20, 129 (1947). - Literature value 219-220°C, see above reference.
Toxicity and behavioral studies with mice could not distinguish between the first three members of this series. For all, LD50's were established to be between 200-300 mg/kg, i.p., and at 80 mg/kg there was the mild, agitated behavior previously described1; complete recovery occurring within 8 h.
In human subjects, the potency of trimethoxyamphetamine (I, R = CH3) has been established as over twice that of mescaline2. Evaluation of the immediate homologue, α-ethyl mescaline (I, R = ethyl) at oral levels in excess of 2.5 mg/kg (as the free base), produced neither central activity nor psychic disturbance, thus demonstrating a psychotomimetic potency of less than half that of trimethoxyamphetamine, and suggesting that three carbons represent an optimum chain length.