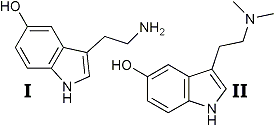
Interest in the physiological actions of tryptamine derivatives has been stimulated considerably by the proposals of Woolley and Shawl and Gaddum2 that serotonin (I) may play a role in central nervous system function. The possibility that the remarkable hallucinogenic effects of lysergic acid diethylamide may be due to its effect as a serotonin antimetabolite has been proposed.1,2 These considerations have been complicated by the observations of Stromberg3 and Evarts4 which indicate that bufotenine (II) is itself a hallucinogenic agent5. Apparently, in South America the native use of bufotenine, from the plant piptadenia peregrina,3,6 has been in the past as widespread as the peyote cult of the North American West.7
To obtain bufotenine and its relatives in quantity for study of their central nervous system effects, a new tryptamine synthesis has been developed which appears to be of wide scope and general application. Giua8,9 has claimed that oxalyl chloride reacts with indole to give 2-indoleglyoxylyl chloride. A reinvestigation of these apparently neglected studies has disclosed that the beautifully crystalline product, which is obtained in practically quantitative yield, is 3-indoleglyoxylyl chloride. Giua based his erroneous structure assignment on the observation that 2-indolecarboxylic acid was isolated from a potassium hydroxide fusion of the supposed 2-indoleglyoxylic acid obtained from the acid chloride. Such evidence has been shown to be of doubtful value as a similar alkali fusion led Asahina and Mayeda10 to incorrect structure proposals for the alkaloids rutaecarpine and evodiamine. As early as 1888 Ciamician et al.,11 observed that skatole gave 2-indolecarboxylic acid (mp 203-204°C) on alkali fusion.
Proof of the position of the glyoxylyl chloride substituent on the indole ring was obtained in an unexpected manner when it was observed in our laboratories that lithium aluminum hydride reduction of the amide obtained from the acid chloride and ammonia yielded tryptamine [3-(2-aminoethyl)-indole], while a similar reduction of the ethyl ester of the glyoxylic acid yielded tryptophol [3-(2-hydroxyethyl)-indole]12. Also, a sample of ethyl 3-indoleglyoxylate which was obtained by the method of Oddo and Albanese13 from indole magnesium iodide and ethyl oxalyl chloride did not depress the melting point (183-185°C) of the ester we obtained from the indoleglyoxylyl chloride.
We have found the reaction of oxalyl chloride with indoles offers an attractive approach to a variety of indoleglyoxylic acid derivatives and tryptamines. The reaction has been found to be quite general in application as nicely crystalline glyoxylyl chloride derivatives have been obtained from 2-methylindole, 2-phenylindole, 5,6-dimethoxyindole, 5-acetoxyindole, 5-benzyloxyindole, 6-acetoxy-7-methoxyindole and 1-benz[g]indole. Ethyl 2-indolecarboxylate was unaffected by oxalyl chloride. The excellent yields of amides obtained from the glyoxylyl chlorides together with the facile conversion of the amides to tryptamines in good yield with lithium aluminum hydride have established this route to derivatives of 3-(2-aminoethyl)-indole to be the most convenient of those thus far studied.14
This method has been extended to the preparation of the blood serum vasoconstrictor agent serotonin (5-hydroxytryptamine).15 5-Benzyloxyindole reacted with oxalyl chloride to give a practically quantitative yield of crude 5-benzyloxy-3-indoleglyoxylyl chloride (mp 146-150°C, dec.). The acid chloride with dibenzylamine gave a 91% yield of 5-benzyloxy-3-indole-N,N-dibenzylglyoxylamide melting at 150-151°C. When this amide was reduced with lithium aluminum hydride 5-benzyloxy-3-(2-dibenzylaminoethyl)-indole was isolated in 92% yield as the hydrochloride salt, melting at 232-233°C.
This amine was converted to the free base and catalytically debenzylated. The creatinine sulfate complex obtained from the resulting base was identical with the serotonin complex prepared by an earlier method16. For the preparation of bufotenine, 5-benzyloxy-3-indoleglyoxylyl chloride was treated with dimethylamine to obtain 5-benzoyloxy-N,N-dimethyl-3-indoleglyoxylamide, mp 178-180.5°C. With lithium aluminum hydride the glyoxylamide gave 5-benzyloxy-3-(2-dimethylaminoethyl)-indole which was isolated as the hydrochloride salt, mp 154-155°C. Debenzylation of the free base obtained from the above hydrochloride salt gave bufotenine base, mp. 146-147°C. The picrate of this base has been shown to be identical to the bufotenine picrate obtained from pipladenia peregrina3.