Ayahuasca: alkaloids, plants & analogs
Section 1 :
Structure & properties of Tetrahydroharmine
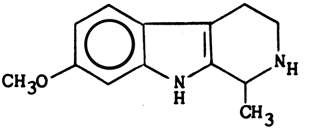
C,13H16N2O
MW 216.28 Ott 1996
MW 216.282 Southon & Buckingham 1989.
C 72.19%, H 7.46%, N 12.95%, O 7.40% Ott 1996
Legal Status: Tetrahydroharmine lacks abuse potential and is not a controlled substance.
[Appears to be watched. Ott 1996]
Free base:
mp 187-190° (White crystals from methanol) Shulgin & Shulgin 1997
mp 190-195° Bernauer 1964
mp 195-198° Straw-colored microneedles from xylene with traces of ether.
Ghosal et al. 1971
mp 198.4-199.8° (in vacuo). mmp with racemic (same mp in vacuo) was not depressed. Hochstein & Paradies 1957
mp 198.4-199.8° (R-Form)
Southon & Buckingham 1989
mp 199° Perkin & Robinson 1919, page 961 [Also Manske 1965 citing Fischer 1889]
mp 199° (Slender colorless needles from methanol-benzene) Siddiqui et al. 1983
mp 199-200° Manske 1965
mp 199-200° Telezhenetskaya 1977
Sublimes in vacuum.
Hochstein & Paradies 1957
Vacuum distillation (in a Kugelrohr):
0.001 Torr 120-150° distillation
Bernauer 1964
Soluble in chloroform, ethanol & methanol. Ott 1996.
Soluble in ethyl acetate. Siddiqui et al. 1983
Optical rotation:
[α]25D +32° (CHCl3 or 5% acetic acid). Hochstein & Paradies 1957 and
Southon & Buckingham 1989 [Note 1]
[α]25D +28° (c, 0.516; CHCl3)
Ghosal et al. 1971
(±)-form (Synthetic)[The optically inactive racemate also occurs in nature.]
CA Reg. 46501-01-2
Needles from methanol mp 198-199°
Southon & Buckingham 1989
Hochstein & Paradies 1957 reported that the R-form and synthetic racemic mixture had identical IR and paper chromatographic results.
Assay: [Fluorescent under UV; see also Chromophore list below.]
Fluorescence: Yes.
Fluorescence maxima of 355 nm (in ethanol). Agurell et al. 1969
Fluoresces (in CHCl3) from 480 to 700 nm
Bernauer 1964
UV: Paris et al. 1957. [Isol, struct, uv, ir]
UV λmax 217, 268-272 nm (log ε 4.53 and 3.72) Ghosal et al. 1971. [Ed.: µ=nm]
UV λmax 225 nm, 269 nm, 296 nm (logε 4.52, 3.69 and 3.75)
Bernauer 1964
IR:
Paris et al. 1957. [Isol, struct, uv, ir]
IR and MS:
Shulgin & Shulgin 1997.
MS:
Holmstedt & Lindgren 1967 and
Ghosal et al. 1971.
CMR: Ghosal 1972.
Isolations:
Hochstein & Paradies 1957 [As d-THH; from a previously prepared brew thought to be from Banisteriopsis caapi but unvouched.]
Paris et al. 1955
Paris et al. 1957 [As dl-THH from Leptactinia densiflora.]
Synthesis:
First prepared by Fischer 1889 [Preparation 1] and 1897 [Preparation 2]
Examples of published preparations:
1. Harmine or Harmaline reduced in alcoholic solution by Sodium.
2. Harmine or Harmaline reduced in Isoamyl alcohol solution by Sodium.
3. Harmaline (5 grams) dissolved in 100 cc H2O and 20 cc HCl , heated to boiling and then Sodium amalgam added (with HCl).
4. Perkin et al. 1919 [Synthetic]
5. Harmaline reduced in methanol with NaBH4.
See also Shulgin & Shulgin 1997.
6. Harmaline has also been reduced to THH using Zinc &Hydrochloric acid [Note 2]
. Siddiqui et al. 1983 (This should also work for harmine) They found that addition of NH4Cl prior to basification with Ammonia increased the yield for this reaction from 50% to around 80%.
7. 8-Nitroharmidine reduced with NaBH4. (THH was obtained with around a 90% yield) Siddiqui et al. 1983
Structure: Paris et al. 1957
Absolute configuration:
Koblicová & Trojánek 1966.
Chromatography:
Liquid:
See comments elsewhere here on the separation of a mixture of these alkaloids including THH.
CHCl3-MeOH was used to elute this compound from Florisil. (Introduced with benzene. Elution with Chloroform, Chloroform-Methanol and then Methanol.)
Ghosal et al. 1971.
| tlc: | |
| Solvent system | Rf |
| Methyl alcohol-Chloroform-Acetic acid (75:25:15) |
0.66 |
| Ghosal et al. 1971 | [Silica Gel G chromatoplates] |
| Chloroform-Formamide | 0.13 |
| Hochstein & Paradies 1957 citing |
Hochstein et al. 1955 |
| Acetone-Ethanol (85:15) | 0.13 |
| Bernauer 1964 | [on Alumina] |
| Chromophores of Tetrahydroharmine | |
| α-Nitroso-β-naphtholnitrous acid | Dull violet |
| Dragendorff's | Orange |
| Fröhde | Navy blue |
| Hopkin-Cole | Purple |
| Ehrlich's | Negative [Ed.: Slow; not negative. See below.] |
| Ghosal et al. 1971. | [all on silica gel] |
Unlike the tryptamines which react within 30 minutes and develop a dark blue color, THH produces a characteristic robin's egg blue over a 24 hour period.
McKenna et al. 1984.
Hydrochloride is colorless.
Perkin & Robinson 1919 (page 961)
mp 232-234° (Fine solid with greenish tinge) Shulgin & Shulgin 1997
IR and MS of tetrahydroharmine hydrochloride: Shulgin & Shulgin 1997
Notes #
- Southon & Buckingham stated that all their physical constants referred to the alkaloid as isolated from B. caapi and that specific rotations of the isolates from other species were not available. It might be noted that Paris et al. reported the natural occurrence of the racemate (optically inactive) in Leptactinia densiflora and Ghosal 1972a reported the (+)-form in the stem and leaf of Banisteriopsis argentea.
- Unanswered question. This is apparently based on a single trial involving one individual. There appears to be no account of anyone who has actually evaluated the pure material as an activator for the active tryptamines. Based on the composition of some active ayahuasca brews involving the Cielo cv. there is every indication that it possibly might work despite the lack of good MAOI properties but this appears to be untested and unproven. (Said Cielo cv. brews contain what would seem to be inadequate amounts of harmmine but high levels of THH.)


