
#63 DOBU
2,5-DIMETHOXY-4-(n)-BUTYLAMPHETAMINE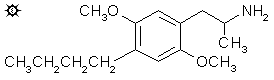
|
| [3D .mol structure] |
To 150 g mossy zinc there was added a solution of 3 g mercuric chloride in 60 mL H2O, and this was swirled periodically for 2 h. The H2O was drained off, and the amalgamated zinc added to a 1 L three-neck round-bottomed flask, treated with 80 mL concentrated HCl, and heated on the steam bath. A solution of 20.8 g of 2,5-dimethoxybutyrophenone in 45 mL EtOH containing 10 mL concentrated HCl was added in increments over a 4 h period. During this period an additional 140 mL of concentrated HCl was added periodically to the ketone solution. Heating was maintained for an additional 4 h. After cooling, the aqueous filtrate was extracted with 3x100 mL CH2Cl2 and these pooled extracts washed with 2x200 mL 5% NaOH to remove a small amount of phenolic impurity. After removal of the solvent under vacuum, the residual 16.1 g of clear oil was distilled over the 100-160 °C range (largely at 141-145 °C) at the water pump to give 10 g of 2,5-dimethoxy-(n)-butylbenzene as a white oil. This was about 90% pure by GC analysis, and was used without further purification in the next step.
A mixture of 98 mL POCl3 and 108 mL N-methylformanilide was allowed to incubate for 0.5 h. To this there was then added 47.3 g of 2,5-dimethoxy-(n)-butylbenzene and the mixture heated on the steam bath for 1.5 h. This mixture was poured into 1 L H2O and stirred overnight. The H2O was drained from the extremely gooey black crystals that were formed, and extracted with 2x100 mL portions of hexane. The black residue was diluted with these extracts and, on slow evaporation there was deposited 26.4 g of oily amber crystals. Filtering these through a medium porous funnel and sucking the oily phase away from the solids yielded 14.8 g of yellow crystals that could be recrystallized from 50 mL MeOH to give, after filtration and air drying to constant weight, 6.4 g of 2,5-dimethoxy-4-(n)-butylbenzaldehyde as pale yellow crystals with a mp of 47-48 °C. The recovery of all organic soluble things from the above process gave, after removal of the extraction solvents and making boiling hexane extractions of the residues, a second crop of aldehyde of equal weight and of identical mp. An analytical sample, from hexane, had the same mp. Anal. (C13H18O3) C,H.
A solution of 13.2 g 2,5-dimethoxy-4-(n)-butylbenzaldehyde in 50 mL acetic acid was treated with 4.0 g anhydrous ammonium acetate and 10 mL nitroethane. This mixture was heated on the steam bath for 4 h, then poured into a large quantity of H2O. This was extracted with 2x200 mL CH2Cl2, the extracts washed with H2O, and the solvent removed to give 19 g of a deep red oil. This was dissolved in 35 mL hot MeOH and slowly cooled, depositing yellow-orange crystals. These were removed by filtration, washed with cold MeOH, and air-dried to constant weight. Thus there was obtained 11.8 g of 1-(2,5-dimethoxy-4-(n)-butylphenyl)-2-nitropropene with a mp of 54-56 °C. Recrystallization of an analytical sample from MeOH tightened the mp to 55-56 °C. Anal. (C15H21NO4) C,H,N.
To a gently refluxing suspension of 8.5 g LAH in 300 mL anhydrous Et2O under a He atmosphere, there was added 11.0 g 1-(2,5-dimethoxy-4-(n)-butylphenyl)-2-nitropropene by allowing the condensing ether to drip into a Soxhlet thimble containing the nitrostyrene, thus effectively adding a warm saturated solution of it dropwise. Refluxing was maintained overnight, and the cooled reaction flask stirred for several additional days. The excess hydride was destroyed by the cautious addition of 600 mL H2O containing 55 g H2SO4. When the aqueous and Et2O layers were finally clear, they were separated, and 250 g of potassium sodium tartrate was dissolved in the aqueous fraction. Aqueous NaOH was then added until the pH was above 9, and this was then extracted with 3x200 mL CH2Cl2. Evaporation of the solvent produced 12 g of an amber oil that gelatinized to a waxy, amorphous mass. This was leached as thoroughly as possible with anhydrous Et2O which was clarified by filtration, then saturated with anhydrous HCl gas. After a few minutes delay, there commenced the separation of fine white crystals of 2,5-dimethoxy-4-(n)-butylamphetamine hydrochloride (DOBU). These weighed, after filtration, Et2O washing, and air drying to constant weight, 5.8 g. Recrystallization from boiling CH3CN (this is an unusually exothermic crystallization) yielded 5.4 g of a fluffy white product with mp 151-152 °C. Anal. (C15H26ClNO2) C,H,N.
DOSAGE: uncertain.
DURATION: very long.
QUALITATIVE COMMENTS: (with 2.2 mg) It was almost the fourth hour before I noticed something. Then I felt an increasing manic intoxication, winding up tighter and tighter. Sleep was impossible until some 18 hours after the start of the trial. There was some paresthesia, but no mydriasis. This might be a stimulant, but it is not a psychedelic, at least at this level. Go up slowly.
(with 2.8 mg) Nothing for over seven hours. Then there was what seemed to be an irritability and shortness of temper. Mentally I am completely clear, but no more alert than usual. There was no sleep that evening, and the next day there was a feeling of overall depression. Perhaps that was due to the lack of sleep, but there were no signs of residual sleepiness.
EXTENSIONS AND COMMENTARY: It is not possible to give a dosage range for DOBU. There is no question but that whatever is occurring is slow of onset, and very long lived. In general, the effects resemble stimulation more that anything else.
A butyl group has four carbons, and they can be interconnected in four ways (as long as you don't connect them in rings). If all four of them are in a straight chain, you have the so-called normal butyl (or n-butyl) group, and this is the exact arrangement that is found in the DOBU. The atoms can be numbered #1 through #4, going outwards from the point of attachment. The chain can, however, be only three carbons long, and the fourth or extra carbon attached on the #2 carbon atom; this is called the iso-butyl (or i-butyl) group. Or the extra left-over carbon can be attached to the #1 carbon atom; this is called the secondary butyl (or sec-butyl or s-butyl) group. Or lastly, the atoms can be all scrunched up, with the chain only two carbons long, and the other two left-over methyl carbons attached to the #1 carbon atom. This isomer is called the tertiary butyl (or tert-butyl or t-butyl) group. In animal studies, and in preliminary human studies, the activity of these compounds drops as the butyl group gets more and more scrunched.
The isomer with the iso-butyl group has been synthesized by the Friedel- Crafts reaction of isobutyryl chloride with p-dimethoxybenzene, followed by reduction of the ketone to an alcohol, dehydration to a dimethylstyrene, and final hydrogenation to a hydrocarbon. The formation of the benzaldehyde, reaction with nitroethane, and final lithium aluminum hydride reduction to 2,5-dimethoxy-4-(2-methylpropyl)-amphetamine hydrochloride (DOIB, mp 164-166 °C) were completely conventional. In drug discrimination studies in rats, DOIB was only a third as active as DOM, and in humans the activity falls in the 10 to 15 milligram area. The isomer with the sec-butyl group was made in a somewhat similar manner, from 2,5-dimeth-oxyacetophenone. The addition of ethyl magnesium bromide gave an alcohol which with dehydration yielded a pair of dimethylstyrenes isomeric to the compound mentioned above. From there an identical sequence of steps (hydrogenation, benzaldehyde synthesis, nitrostyrene, and lithium aluminum hydride reduction) produced 2,5-dimethoxy-4-(1-methylpropyl)amphetamine hydrochloride (DOSB, mp 168-170 °C.). In the rat studies it was only a twelfth the potency of DOM, and in man the active dose is in the 25 to 30 milligram area. As with the normal butyl compound, there is a strong stimulation factor, with real and long-lasting sleep disturbance.
The last of the butyl isomers, the tert-butyl compound, was made from a much more obvious starting material. This is the commercially available tert-butyl hydroquinone. It was methylated in sodium hydroxide with methyl iodide, and then carried through the above sequence (benzaldehyde. mp 124 °C from cyclohexane, nitrostyrene, yellow crystals from methanol, mp 95-96.5 °C, and lithium aluminum hydride reduction) to give 2,5-dimethoxy-4-(1,1-dimethylethyl)amphetamine hydrochloride (DOTB, mp 168 °C). Rats trained in a process called the Sidman Avoidance Schedule gave behavior that suggested that DOTB had no activity at all, and in human trials, doses of up to 25 milligrams were totally without effect.
An effort was made to prepare a butyl analogue containing a ring, but it was never completed. This was the cyclopropylmethyl isomer, 2,5-dimethoxy-4-cyclo-propylmethylamphetamine hydrochloride, DOCPM. Only the first step of its synthesis was complete (the reaction of cyclopropylcarboxylic acid chloride with p-dimethoxybenzene) and even it went badly. The desired ketone (2,5-dimethoxyphenyl cyclopropyl ketone) was most difficult to separate from the recovered starting ether. A promising approach would be the isolation of the phenol (2-hydroxy-5-methoxyphenyl cyclopropyl ketone) which is a beautiful yellow solid with a melting point of 99-100 °C from methanol. Anal. (C11H12O3) C,H. It then could be methylated to the wanted intermediate. It is the major product when the reaction is conducted with anhydrous aluminum chloride in methylene chloride.
The 2-carbon phenethylamine homologues of these compounds could all, in principle be easily made by using nitromethane instead of nitroethane with the intermediary benzaldehydes. But, as of the present time, none of them have been made, so their pharmacology remains completely unknown.
| [ |
[Main Index] | [Forward |

