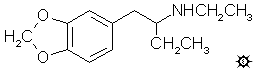
#77 ETHYL-J
2-ETHYLAMINO-1-(3,4-METHYLENEDIOXYPHENYL)BUTANE; N-ETHYL-1-(1,3-BENZODIOXOL-5-YL)-2-BUTANAMINE
SYNTHESIS: A stirred solution of 9.0 g
1-(3,4-methylenedioxyphenyl)-2-butanone (see the recipe for J for its
preparation) in 150 mL MeOH was treated with 9.0 g ethylamine
hydrochloride, 4.0 g anhydrous NaOAc, and 3.0 g sodium
cyanoborohydride. The pH was maintained between 6 and 7 by the
periodic addition of HCl. After the base formation had stabilized,
there was added an additional 9.0 g ethylamine hydrochloride, 9.0 g
NaOAc and 2.0 g sodium cyanoborohydride. With continuous stirring,
there was HCl added over the course of 1 h until the final pH was
approximately 2. The reaction mixture was poured into 700 mL dilute
NaOH, and extracted with 3x75 mL CH2Cl2. These extracts were pooled,
and back-extracted with dilute H2SO4. This was washed with 2x50 mL
CH2Cl2, then made basic with dilute NaOH and extracted with 2x75 mL
CH2Cl2. Removal of the solvent under vacuum gave a 0.81 g residue
which was dissolved in 10 mL IPA. Neutralization with concntrated HCl
formed white crystals spontaneously. These were diluted with Et2O,
filtered, Et2O washed and air dried to provide 0.85 g
2-ethylamino-1-(3,4-methylenedioxy-phenyl)butane hydrochloride
(ETHYL-J), with mp of 176-177 °C. Anal. (C13H20ClNO2) C,H. The
neutral fraction that remained in the organic phase following the
dilute sulfuric acid extraction, was recovered by removal of the
solvent under vacuum. There was obtained about 5 g of an amber liquid
that was largely 2-hydroxy-1-(3,4-methylenedioxyphenyl)butane.
DOSAGE: greater than 90 mg.
DURATION: probably short.
QUALITATIVE COMMENTS: (with 65 mg) Perhaps aware at 20 minutes.
Definitely aware at 45 minutes. Diffusing to nothing at 3-4 hours.
(with 90 mg) I am somewhere between 1 and +. And everything became
lost in the evening with a couple of glasses of wine and talk that
went on to 3 AM.
EXTENSIONS AND COMMENTARY: And nothing higher has ever been looked at.
If the analogy with the amphetamine counterparts (J with MDA, METHYL-J
with MDMA, and this, with MDE) were to hold up (a drop of about a
third in potency with the lengthening of the chain by a carbon atom),
one might guess that this compound would be an interesting intoxicant,
but probably not until you got up into the area at or above a 200
milligram dose. And that is a lot of chemical for the body to have to
handle. Some day, maybe.