
#171 2-TOM
5-METHOXY-4-METHYL-2-METHYLTHIOAMPHETAMINE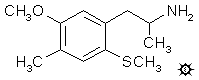
|
| [3D .mol structure] |
To a solution of 25.5 g 2-methyl-4-(methylthio)phenol in 100 mL MeOH there was added a solution of 12 g 85% KOH in 60 mL hot MeOH, followed by the addition of 12.4 mL methyl iodide. The mixture was held at reflux for 16 h. The solvent was removed under vacuum, and the residue added to 400 mL H2O. This was made basic with 25% NaOH and extracted with 3x100 mL CH2Cl2. The extracts were pooled, the solvent removed under vacuum giving 28.3 g of a light, amber oil as residue. This was distilled at 72-80 °C at 0.5 mm/Hg to provide 2-methyl-4-(methylthio)anisole as a pale yellow oil. Anal. (C9H12OS) C,H. The same product can be made with the sulfonyl chloride and the thiol as intermediates. To 36.6 g 2-methylanisole there was added, with continuous stirring, a total of 38 mL chlorosulfonic acid at a modest rate. The exothermic reaction went through a complete spectrum of colors ending up, when the evolution of HCl had finally ceased, as deep amber. When it had returned again to room temperature, the reaction mixture was poured over a liter of cracked ice which, on mechanical stirring, produced a mass of white crystals. These were removed by filtration, washed with H2O, and sucked as dry as possible. The wet weight yield was over 40 g and the mp was about 49 °C. Recrystallization of an analytical sample of 4-methoxy-3-methylbenzenesulfonyl chloride from cyclohexane gave white crystals with a mp of 51-52 °C. A small sample of this acid chloride brought into reaction with ammonium hydroxide produced the sulfonamide which, after recrystallization from EtOAc, melted at 135-136 °C. To a slurry of 300 mL cracked ice and 75 mL concentrated H2SO4 in a round-bottomed flask equipped with a reflux condenser, there was added 43 g of the slightly wet 4-methoxy-3-methylbenzenesulfonyl chloride followed by 75 g elemental zinc dust. The temperature was raised to a reflux which was maintained for 2 h. The reaction mixture was cooled and filtered, with the finely ground filter cake being washed alternately with H2O and with CH2Cl2. The combined mother liquor and washings were diluted with 1 L H2O, the phases separated, and the aqueous phase extracted with 100 mL CH2Cl2 which was added to the organic phase. This was washed with 100 mL H2O, and the solvent removed under vacuum. The residue was a pale amber oil weighing 27.3 g and it slowly set up to a crystalline mass that smelled of banana oil. A portion of this, pressed on a porous plate, gave a waxy solid with a mp of 39-43 °C which, on recrystallization from MeOH, gave 4-methoxy-3-(methyl)thiophenol with a mp of 45-46 °C. Anal. (C8H10OS) C,H. A solution of 24 g of the crude thiol in 100 mL MeOH was treated with a solution of 17 g KOH 85% pellets in 100 mL hot MeOH, and to this there was added 16 mL of methyl iodide. This was held at reflux on the steam bath for 1.5 h, then stripped of solvent under vacuum, added to 1 L H2O, and made strongly basic with 25% NaOH. Extraction with 3x100 mL CH2Cl2, pooling of the extracts, and removal of the solvent, gave an amber oil weighing 22.6 g. This was distilled at 70-80 °C at 0.7 mm/Hg to give 16.3 g of 2-methyl-4-(methylthio)anisole as a white oil, identical in all respects to the product that came from the sulfonium salt pyrolysis above.
A solution of 22.1 g 2-methyl-4-(methylthio)anisole and 17.5 g dichloromethyl methyl ether in 600 mL CH2Cl2 was vigorously stirred, and treated with 24.5 g anhydrous aluminum chloride added portion-wise over the course of 1 min. Stirring was continued for 20 min while the color developed to a dark red. There was added 500 mL H2O with caution, and stirring was continued until the initial yellow solids redissolved and there were two distinct phases formed. These were separated, and the aqueous phase was extracted with 3x100 mL CH2Cl2. The original organic phase and the pooled extracts were combined and washed with 5% NaOH. The organic solvent was removed under vacuum. The residue was distilled, giving two major fractions. A forerun (85-95 °C at 0.5 mm/Hg) proved to be largely starting ether. The major fraction (8.4 g, boiling at 95-120 °C) consisted of two materials, both benzaldehydes. Crystallization of this fraction from 30 mL cyclohexane provided, after filtering, washing and air drying, 2.9 g of 5-methoxy-4-methyl-2-(methylthio)benzaldehyde as a pale yellow crystalline solid with a mp of 69-70 °C. Anal. (C10H12O2S) C,H. The mother liquor from this crystallization contained a slower-moving component, 2-methoxy-3-methyl-5-(methylthio)benzaldehyde, which was best separated by preparative gas chromatography. The proof of the structure of the major aldehyde above was obtained by its reductive conversion to 2,5-dimethyl-4-(methylthio)anisole with amalgamated zinc and HCl. The details are given in the recipe for 5-TOM.
To 4 mL glacial acetic acid there was added 1.0 g 5-methoxy-4-methyl-2-(methylthio)benzaldehyde, 0.35 g anhydrous ammonium acetate, and 0.8 g nitroethane, and the mixture was heated on the steam bath for 4 h. Another 0.5 g of nitroethane was added, and the heating continued for an additional 4 h. Standing at room temperature overnight allowed the deposition of spectacular orange crystals which were removed by filtration, washed lightly with acetic acid, and air dried. This product melted at 82-83 °C. Recrystallization from 10 mL boiling MeOH gave 0.7 g of 1-(5-methoxy-4-methyl-2-methylthiophenyl)-2-nitropropene with a mp of 83-84 °C. Anal. (C12H15NO3S) C,H. The alternate method for the formation of nitrostyrenes, the reaction of the benzaldehyde in nitroethane as both reagent and solvent, with ammonium acetate as a catalyst, gave a gummy product that could be purified only with severe losses. The overall yield with this latter method was 24% of theory.
A solution of 1.5 g LAH in 75 mL THF was cooled, under He, to 0 °C with an external ice bath. With good stirring there was added 1.0 mL 100% H2SO4 drop-wise, to minimize charring. This was followed by the addition of 3.0 g 1-(5-methoxy-4-methyl-2-methylthiophenyl)-2-nitropropene in 20 mL anhydrous THF. After a few min further stirring, the temperature was brought up to a gentle reflux on the steam bath, and then all was cooled again to 0 °C. The excess hydride was destroyed by the cautious addition of IPA followed by sufficient 5% NaOH to give a white granular character to the oxides, and to assure that the reaction mixture was basic. The reaction mixture was filtered, and the filter cake washed first with THF and then with IPA. The filtrate was stripped of solvent under vacuum providing a light yellow oil. This was dissolved in 100 mL dilute H2SO4 and then washed with 2x50 mL CH2Cl2. The aqueous phase was made basic with 5% NaOH and extracted with 2x50 mL CH2Cl2. These were pooled, the solvent removed under vacuum, and the residue distilled at 105-130 °C at 0.25 mm/Hg to give 1.6 g of a white oil. This was dissolved in 8 mL IPA, neutralized with 24 drops of concentrated HCl which formed crystals spontaneously. Another 20 mL of hot IPA was added to effect complete solution, and then this was diluted with anhydrous Et2O. On cooling fine white crystals of 5-methoxy-4-methyl-2-methylthioamphetamine hydrochloride (2-TOM) separated. These weighed 1.55 g and had a mp of 195-196 °C. Anal. (C12H20ClNOS) C,H.
DOSAGE: 60 - 100 mg.
DURATION: 8 - 10 h.
QUALITATIVE COMMENTS: (with 60 mg) There is a superb body feeling, and food tasted excellent but then it just might have been excellent food. By the tenth hour, there were absolutely no residues, and I had the feeling that there was no price to pay. Venture up a bit with confidence.
(with 80 mg) For me this was excellent, in a down-to-earth, humorous, matter-of-fact universe-perspective sense. Very pleasant feeling, although there was a strong body awareness below the waist (not the erotic thing, but rather a slight heaviness, and the next day I came down with a G.I. cold). Very good feeling, and I sense that the depth of the experience is way out there where the big questions lie. I found it easy to go out of body (in the good sense) into a warm, loving darkness. Sliding down by 6, 7th hour, and had no trouble sleeping. Fully scripted dreams, vivid. Very, very good. Want to try 100 mg.
(with 80 mg) Completely foul taste. The effects were quite subtle, and I found this to be a strange but friendly ++. There was much eyes-closed fantasizing to music, even to Bruchner, whom I found unexpectedly pleasant. There was a feeling of tenseness at the twilight of the experience.
EXTENSIONS AND COMMENTARY: There is a most extraordinary loss of potency with the simple substitution of a sulfur atom for an oxygen atom. DOM is fully active at the 5 or so milligram area, whereas 2-TOM is active at maybe the 80 milligram area, a loss of potency by a factor of x15 or so. And the duration is quite a bit shorter. It might take a fair amount of learning to become completely at peace with it, but it might be worth the effort. And there are none of the disturbing hints of neurological and physical roughness of 5-TOM.
Again, as with the other TOM's and TOET's, the two-carbon homologue of this has been synthesized but not yet evaluated. The common intermediate benzaldehyde, 5-methoxy-4-methyl-2-(methylthio)benzaldehyde was condensed with nitromethane and ammonium acetate to give the nitrostyrene which, upon re-crystallization from ethanol, had a melting point of 118-118.5 °C. Anal. (C11H13NO3S) C,H. Reduction with aluminum hydride in THF gave the crystalline free base which, as the hydrochloride salt, melted at 233-234 °C. Anal. (C11H18ClNOS) C,H. Quite logically, it has been called 2C-2-TOM.
| [ |
[Main Index] | [Forward |

