NIH Kava Chemistry & Toxicology Executive Summary
Nov 1998
Original URL: http://ntp-server.niehs.nih.gov/htdocs/Chem_Background/ExecSumm/Kava.html (9000-38-8; 84696-40-2)
- Basis for Nomination
- Chemical Identification
- Production Information
- Use Pattern
- Human Exposure
- Regulatory Status
- Evidence for Possible Carcinogenic Activity
- References
BASIS OF NOMINATION TO THE CSWG
Kava kava is brought to the attention of the CSWG because it is a rapidly growing, highly used dietary supplement introduced into the mainstream U.S. market relatively recently. Through this use, millions of consumers using antianxiety preparations are potentially exposed to kava kava.
A traditional beverage of various Pacific Basin countries, kava clearly has psychoactive properties. The effects of its long-term consumption have not been documented adequately; preliminary studies suggest possibly serious organ system effects. The potential carcinogenicity of kava and its principal constituents are unknown.
INPUT FROM GOVERNMENT AGENCIES/INDUSTRY
The U.S. Pharmacopeia is in the process of reviewing kava kava. No decision on preparation of a monograph has been made.
SELECTION STATUS ACTION BY CSWG: 12/14/98
Studies requested:
- Toxicological evaluation, to include studies of reproductive toxicity and neurotoxicity
- Genotoxicity
Rationale/Remarks:
- Significant human exposure
- Leading dietary supplement with rapidly growing use
- Concern that kava has been promoted as a substitute for ritilin in children
- Test extract standardized to 30 percent kavalactones
- NCI is conducting studies in Salmonella typhimurium
CHEMICAL IDENTIFICATION
Description: The tropical shrub Piper methysticum is widely cultivated in the South Pacific. The name kava refers to the plant, the beverage prepared from the plant, and the ceremony associated with drinking the beverage; variants of the name include awa and kawa (Smith et al., 1984; Lebot et al., 1992; Dentali, 1997).
CAS Registry Number: 9000-38-8 Chemical Abstract Service Name: Kava-kava resin (8CI) CAS Registry Number: 84696-40-2 Chemical Abstract Service Name: Pepper (Piper), P. methysticum, ext. Synonyms and Trade Names: Extract of kava; kava extract; Piper methisticum extract
The kava plant is a hardy, fairly succulent, slow-growing perennial belonging to the Piperacae family. When cultivated, the plant is usually harvested when it is about 2-2.5 meters tall. The leaves are heart-shaped, pointed, quite long (8-25 cm), and smooth and green on both sides. Kava is cultivated for its rootstock, also referred to as the stump. The stump is knotty, thick, and sometimes tuberous, and it often contains holes or cracks created by partial destruction of the parenchyma. A fringe of lateral roots up to three meters long extends from the pithy rootstock. The roots comprise a multitude of ligneous fibers and consist of more than 60 percent starch. Rootstock color varies from white to dark yellow, depending upon the amount of kavalactones that are contained in the lemon-yellow resin (Lebot et al., 1992; Singh, 1992).
Kava cultivation and selection has produced numerous varieties or cultivars recognized by differences in the internodes (space between stem joints), color of stems, intensity of leaf color, and quality of the root. Different varieties are classified, named, and used for different purposes by the native people. Individual kava cultivars are selected on the basis of subjective appraisal of the effect produced by consumption of a particular plant (Dentali, 1997).
Chemical Composition: Analysis of the composition of kava rootstock indicates that fresh material on average is 80 percent water. When dried, rootstock consists of approximately 43 percent starch, 20 percent fibers, 12 percent water, 3.2 percent sugars, 3.6 percent proteins, 3.2 percent minerals and 15 percent kavalactones, although the kavalactone component can vary between 3 percent and 20 percent of rootstock dry weight depending on the age of the plant and the cultivar. The active principles of kava rootstock are mostly, if not entirely, contained in the lipid-soluble resin. The isolates of kava resin fall into three general categories: arylethylene-a pyrones, chalcones and other flavanones, and conjugated diene ketones. The compounds of greatest pharmacological interest are the substituted a -pyrones or kava pyrones, commonly called kavalactones. Fifteen lactones have been isolated from kava rootstock. The following six compounds are present in the highest concentrations and account for approximately 96 percent of the lipid resin: yangonin, methysticin, dihydromethysticin, kavain, dihydrokavain, and demethoxyyangonin (5,6-dehydrokavain). Structures for the 15 kavalactones are shown in Table 1 (Shulgin, 1973; Lebot et al., 1992; Dentali, 1997).
Technical Products and Impurities: Kava products, standardized to contain 30 percent kavalactones are available at health food stores and pharmacies as well as through direct-mail companies. In the US market, kava root is supplied as crude herb as well as in the form of tinctures, powdered extracts, and standardized extracts. Extracts prepared from stem peelings and other above-ground plant parts are also available, these are reportedly the source for some European standardized products (Dentali, 1997).
Some of the many commercially-available kava products include:
Capsules. Capsules range in strength from 80 to 250 mg and most are standardized to contain 30 percent kavalactones (Elixir, 1997; Reach4Life, 1997; Herbal Alternates, 1998).
Table 1. Fifteen Kavalactones
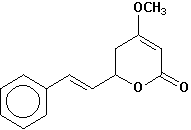 kavain |
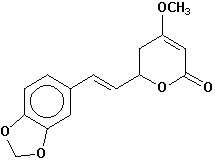 methysticin |
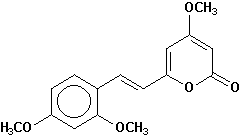 10-methoxyyangonin |
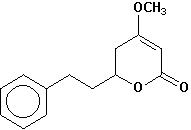 7,8-dihydrokavain |
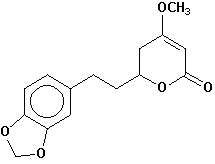 dihydromethysticin |
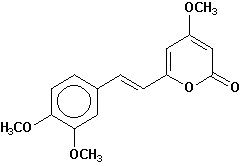 11-methoxyyangonin |
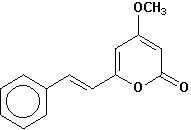 5,6-dehydrokavain |
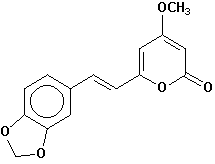 5,6-dehydromethysticin |
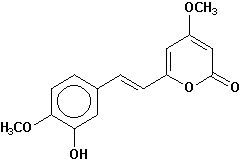 11-hydroxyyangonin |
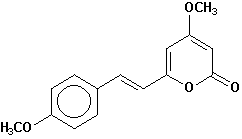 yangonin |
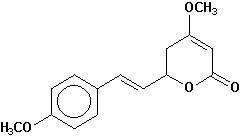 5,6-dihydroyangonin |
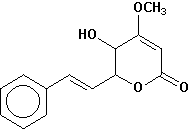 hydroxykavain |
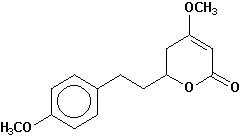 5,6,7,8-tetrahydroyangonin |
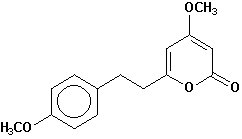 7,8-dihydroyangonin |
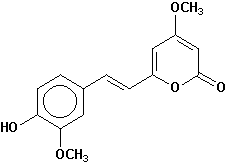 11-methoxy-12-hydroxydehydrokavain |
Powder/Tea. Botanicals International (1998) offers kava kava rhizome powdered extract standardized to contain >30 percent kavapyrones. Kava King (1998) offers powdered kava in plain, cocoa, and strawberry flavors and traditional kava (slight vanilla flavor) to prepare as tea; each tablespoon of these products has 200 mg of kavalactones.
Liquid. Kava root extract in 50-58 percent pure grain alcohol and vegetable glycerine is available from Vitanet; 10 drops of the liquid contain 75 mg of kavalactones (Vitanet, 1998).
Kava is also available in combination products which contain a variety of herbs and/or vitamins (Herbal Resources, 1995; Femhealth, 1998; Nutrahance, 1998; Reach4Life, 1998; Ripplecreek, 1998).
EXPOSURE INFORMATION
Production and Producers: Although P. methysticum does flower, it is incapable of sexual reproduction and propagation is dependent on cultivation. Farmers plant cuttings taken from existing stems and new growth occurs at a stem bud at the axil of a lateral branch scar (Lebot et al., 1992).
In the South Pacific, the preparation of the kava root and the consumption of the kava beverage were highly ritualized. The beverage was prepared by mixing crushed, grated, or chewed kava root with water or coconut milk. In Micronesia and parts of Melanesia, the roots were pounded or grated. In Polynesia, the roots were chewed and the macerated pulp was expectorated into a communal bowl, infused with liquid, and strained through plant fibers. The chewing method is rarely used in modern Polynesia, instead kava roots are pulverized into powder with makeshift mortars and pestles (Norton & Ruze, 1994). It was once thought that the action of saliva on kava root was important to release its pharmacological effect. It is now accepted that the lipid soluble material is released into aqueous solution through emulsification and that this process is mechanical, not enzymatic or chemical (Dentali, 1997).
Kava resin, which contains the biologically-active kavalactones, can be extracted with organic solvents. Jamieson and coworkers (1989) extracted ground commercial preparations of P. methysticum, with dichloromethane. The extraction was repeated twice, the filtrates combined, dried over anhydrous sodium sulfate, the organic phase filtered, and the solvent removed. The resulting oil, kava resin, was obtained in a yield of 6 percent.
Chemical synthesis of the following kavalactones has been reported: kavain, dihydro-kavain, dihydromethysticin, methysticin, 5,6-dehydromethysticin, demethoxyyangonin, 11-methoxyyangonin, 11-methoxynoryangonin, and yangonin (Israili & Smissman, 1976; Lebot et al., 1992).
Kava kava is available from CPB International, Inc., M.W. International, Inc., and Maypro Industries, Inc. Kava kava extract is available from Ashland Chemical Co., Mini Star International, Inc., and QBI (Quality Botanical Ingredients, Inc.). Botanical Products International, Inc. offers both kava kava extract and tincture (McCoy, 1997).
In the 11-month period from August 1997 to July 1998, the Port Import/Export Reporting Service (PIERS) database reported kava kava imports of 226,437 pounds including 186,289 pounds of kava kava peelings, 24,716 pounds of root powder, and 15,432 pounds of roots (Dialog, 1998).
In 1998, kava kava was the fifth leading seller in the North American botanicals market with estimated sales of $106 million (Mirasol, 1998).
Kava kava is not listed in the EPA’s Toxic Substances Control Act (TSCA) Inventory.
Use Pattern: Kava is a psychoactive beverage used socially and ceremonially for thousands of years by many Pacific Island societies. Events typically accompanied by kava ceremonies included weddings, funerals, births, religious occasions, seasonal feasts, reconciliations, welcoming of important visitors, and exchanging of gifts. Although usually restricted to use by royalty and priests, in some cultures commoners were permitted to drink kava for physical and emotional relaxation after work. In most kava societies, use by women was, and still is, unacceptable. Today kava continues to occupy a central place in everyday life in the islands concerned, although its role has been somewhat diminished. In some societies kava can now be drunk informally at kava pubs (Singh, 1992; Norton & Ruze, 1994).
Since it does not produce the violent behavior associated with alcohol abuse, kava was introduced in Aboriginal communities of northern Australia in the early 1980s as an alternative to alcohol (Dentali, 1997).
The modern pharmacologic investigation of kava began around 1900; several substances from the roots were used briefly in Europe as diuretics and as a treatment of gonorrhea (Norton & Ruze, 1994). Today the German Commission E Monographs allow kava use for the treatment of nervous anxiety, stress, and restlessness; a daily dosage of herb and preparations equivalent to 60-120 mg kava pyrones is recommended (Blumenthal, 1998).
In the US, kava is promoted as a "natural" alternative to such anxiety drugs as Xanax and Valium. In some circles it has a reputation as both an aid to feeling high and an aphrodisiac. In early 1996, 21 herbal companies formed the Kava General Committee to push the herb into the mainstream by focusing attention on kava’s remedial qualities and playing down its recreational aura (Peterson, 1998).
The herbal medicine industry claims that kava promotes relaxation, induces restful sleep, relieves headache and migraine pain, promotes sociability and relieves fatigue; its diuretic and antiseptic properties are also cited (Norton & Ruze, 1994; JNC Corp., 1997). One kava product claims to help children with hyperactivity (Symmetry, 1998).
Human Exposure: Outside of the traditional use of kava in the South Pacific islands, the primary exposure of humans to the drug occurs though its use as an herbal supplement. Fueled primarily by aging baby boomers looking for natural alternatives to drugs, the herbal market has grown to include almost 30 percent of the US adult population, according to a 1997 Gallup Survey and in 1998, kava was the fifth leading herbal seller in the North American market (Mirasol, 1998; Peterson, 1998). A 20/20 television report on the "Kava craze" noted that while kava remains untested in the US, people are buying and health stores, pharmacies, and Wal-marts are increasing their orders to keep the shelves stocked (ABC News, 1998).
No listing was found for kava in the National Occupational Exposure Survey (NOES), which was conducted by the National Institute for Occupational Safety and Health (NIOSH) between 1981 and 1983.
Environmental Occurrence: Because of its traditional importance and psychoactive properties, voyaging islanders carried the kava plant widely throughout the Pacific. These cultural exchanges led to the establishment of kava in the three ethnogeographic regions of the Pacific: Polynesia, Melanesia, and Micronesia (Norton & Ruze, 1994). Evidence suggests that cultivated kava (P. methysticum) derives from a wild progenitor P. wichmannii (Lebot et al., 1992).
Regulatory Status: Since 1994, dietary supplements have been regulated under the Dietary Supplement Health and Education Act (DSHEA). For dietary supplements on the market prior to October 15, 1994, the DSHEA requires no proof of safety in order for them to remain on the market. The labeling requirements for supplements allow warnings and dosage recommendations as well as substantiated "structure or function" claims. All claims must prominently note that they have not been evaluated by the FDA, and they must bear the statement "This product is not intended to diagnose, treat, cure, or prevent any disease" (Croom & Walker, 1995).
EVIDENCE FOR POSSIBLE CARCINOGENIC ACTIVITY Human Data: No epidemiological studies or case reports investigating the association of exposure to kava and cancer risk in humans were identified in the available literature.
Traditional kava use at frequent high doses causes a reversible dermatological condition known as kava dermopathy (Dentali, 1997). Kava dermopathy is an acquired reversible ichthyosis or scaly skin eruption. It arises after prolonged and excessive kava consumption and appears as a generalized, shiny, scaly skin resembling a cracked porcelain glaze (Norton, 1998). Kava dermopathy is believed to be related to interference with cholesterol metabolism (Ruze, 1990; Dentali, 1997).
Heavy consumption of kava has been reported to lead to adverse health effects in some Australian Aborigines (Cawte, 1986, 1988). Mathews and coworkers (1988) conducted a pilot health survey of 39 kava users and 34 non-users in an Aboriginal community. Twenty respondents were very heavy users of kava (mean consumption 440 g/week), 15 were heavy users (310 g/week) and 4 were occasional users (100 g/week). The study found that in addition to causing acute intoxication, sedation and relaxation, a rash, and weight loss in long-term users, kava may also cause liver and renal dysfunction, hematological abnormalities, and possibly pulmonary hypertension. The conclusion that kava affects the liver was based on the markedly-elevated plasma levels of g -glutamyl transferase observed in kava users. At the time of this study, some Aborigines were consuming levels of kava that were up to 100-times higher than those usually consumed in traditional kava societies (Cawte, 1988).
Schelosky and coworkers (1995) reported the development of clinical signs suggestive of central dopaminergic antagonism in four patients who took kava preparations for anxiety.
Two German clinical studies have examined the anxiolytic effectiveness of WS 1490, a kava extract with a high kava pyrone content equal to 70 percent. Volz and Kieser (1997) included 101 outpatients in a 25-week randomized placebo-controlled double-blind trial.
WS 1490 administered 3 times a day significantly improved anxiety symptoms from week 8 on. Adverse events were rarely reported in either treated or placebo groups; stomach upset, judged "possibly related" to treatment, was noted twice. In another placebo-controlled double-blind study of 29 patients with anxiety syndromes, treatment with 100 mg of WS 1490 three times a day significantly reduced anxiety symptoms after one week; no adverse effects were noted during the 4-week treatment (Kinzler et al., 1991).
Almeida and Grimsley (1996) reported a possible interaction of kava and benzodiazepines in a 54-year-old man hospitalized in a semicomatose state. The authors cite the potential for dangerous interactions between kava and prescription drugs.
Animal Data: Kava resin orally administered to male Balb/c mice produced lethality in more than 50 percent of the mice at doses of 700 mg/kg and above (Jamieson & Duffield, 1990). No further toxicity information was identified for kava; acute toxicity values for the six major kavalactones are shown in Table 2 (NLM, 1998).
|
Table 2. LD50 (mg/kg) values for kavalactones |
||||||
|
Species (route) |
Kavain |
Dihydro- kavain |
Methysticin |
Dihydro- methysticin |
Yangonin |
Demethoxy- yangonin |
|
Mouse |
- |
- |
- |
- |
- |
- |
|
(oral) |
1130 |
920 |
- |
1050 |
>1500 |
>800 |
|
(ip) |
420 |
325 |
530 |
420 |
>1500 |
>800 |
|
(iv) |
69 |
53 |
49 |
- |
41 |
55 |
|
Dog |
- |
- |
- |
- |
- |
- |
|
(ip) |
- |
>200 |
- |
>200 |
- |
- |
|
Cat |
- |
- |
- |
- |
- |
- |
|
(ip) |
- |
>250 |
- |
- |
- |
- |
|
Rabbit |
- |
- |
- |
- |
- |
- |
|
(ip) |
- |
>350 |
- |
300 |
- |
- |
The toxicity of the kavalactone demethoxyyangonin was evaluated in male ICR mice and male Wistar rats. Mice received oral demethoxyyangonin doses of 30, 100, or 300 mg/kg twice a day for 14 days or 100 mg/kg twice a day for 9 weeks; rats were treated wit 30, 100, or 300 mg/kg daily for 3 months. In all groups, histological and hematological findings were negative. In rats, demethoxyyangonin lowered cholesterol in the first two months but increased cholesterol after three months (Hsu et al., 1994).
Hapke and coworkers (1971) conducted subchronic toxicological studies of KavaformŽ, a German geriatric preparation containing 50 mg of d,1-kavain and 200 mg of magnesium orotate. Male and female Wistar rats received oral Kavaform doses of 10, 100, or 400 mg/kg for 91 days (after 8 weeks the high dose was increased to 1,000 mg/kg); male and female mongrel dogs received oral Kavaform doses of 10,100, or 200 mg/kg for 3 months. Serum glutamate pyruvate transaminase (SGPT) levels were significantly increased in high-dose rats, however, liver cell damage was not confirmed by histological examination. In high-dose dogs, Kavaform was mildly toxic; proliferation of the small cells of the thyroid epithelium and a multicentric necrosis of the parenchyma of the liver were observed as a single histological finding in one high-dose dog.
No 2-year carcinogenicity studies of kava in animals were identified in the available literature.
Short-Term Tests: No information on the mutagenic or genotoxic activity of kava was found in the available literature.
Hsu and coworkers (1994) reported that the kavalactone demethoxyyangonin was negative in the Ames assay however, no details of the study were given.
Metabolism: Duffield and coworkers (1989) identified a complex mixture of metabolites and unchanged kavalactones in human urine following ingestion of kava prepared by the traditional method of aqueous extraction of commercial Piper methysticum. The nine kavalactones identified were dihydrokawain, kawain, desmethoxyyangonin, tetrahydroxyangonin, dihydromethysticin, 11-methoxytetrahydroyangonin, yangonin, methylsticin, and dehydromethylsticin. Observed metabolic transformations included the reduction of the 3,4-double bond and/or demethylation of the 4-methoxyl group of the a pyrone ring system. Demethylation of the 12-methoxy substituent in yangonin (or alternatively hydroxylation at C-12 of desmethoxyyangonin) was also observed.
Rasmussen and coworkers (1979) investigated the metabolism of the 5,6-dihydro-a pyrones, dihydrokawain, kawain, and methysticin, and the a -pyrones, 7,8- dihydroyangonin and yangonin in rats.
Dihydrokawain. Approximately half of an oral dose of dihydrokawain (400 mg/kg) was recovered as metabolites in the urine in 48 hours. Nearly a 2:1 ratio between hydroxylated and ring-opened products was seea n. There were three mono- and three di-hydroxlated derivatives, of which p-hydroxydihydrokawain was the most abundant. The remaining third consisted of metabolites formed by scission of the 5,6-dihydro-a -pyrone ring and included hippuric acid. Figure 1 shows metabolic pathways proposed for dihydrokawain, which was the principal test compound in the study.
Small amounts of unchanged dihydrokawain were found in the feces. No metabolites were identified in feces or 0-22 hour bile samples.
Kawain. Although lower amounts of urinary metabolites were excreted following kawain administration, both hydroxylated and ring-opened products were formed. Metabolites identified included p-hydroxybenzoic acid; 4-hydroxy-6-phenyl-5-hexen-2-one; hippuric acid; 4-hydroxy-6-hydroxyphenyl-5-hexen-2-one; p-hydroxydihydrokawain; hydroxykawain; p-hydroxykawain; and p-hydroxy-5,6-dehydrokawain. Two metabolites were unidentified. Large amounts of unchanged compound were identified in the feces.
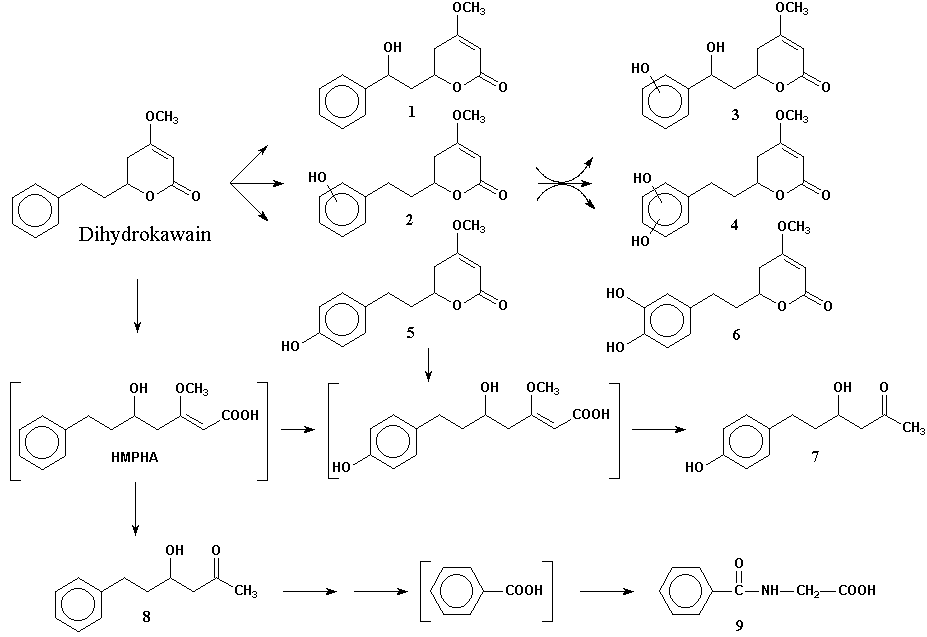
Figure 1. Proposed metabolic pathways of dihydrokawain in the rat
Compounds:
1. 8-hydroxydihydrokawain; 2. hydroxydihydrokawain; 3. dihydroxydihydrokawain; 4. dihydroxydihydrokawain; 5. p-hydroxydihydrokawain; 6. m,p-dihydroxydihydrokawain; 7. 4-hydroxy-6-hydroxyphenylhexan-2-one; 8. 4-hydroxy-6-phenylhexan-2-one; 9. hippuric acid; HMPHA. 5-hydroxy-3-methoxy-7-phenyl-2-heptenoic acid
Methysticin. Methysticin gave rise to only small amounts of two urinary metabolites formed by demethylenation of the methylenedioxyphenyl moiety (m,p-dihydroxykawain and m,p-dihydroxydihydrokawain). Unchanged methysticin was identified in feces.Other Biological Effects: A test mixture of 40 percent kavain, 40 percent dihydrokavain, and 20 percent yangonin was not teratogenic or embryotoxic in Wistar rats; the mixture was administered orally at doses of 100 or 500 mg/kg on days 6-15 of gestation. The mean fetal weight in treated animals was lower than in the controls, although it still fell within physiological limits. The possibility of an effect of the compounds on fetal weight could not be excluded. The mixture was also negative for teratogenic activity in New Zealand strain rabbits when administered orally at doses of 20 or 200 mg/kg on days 6-18 after mating. There was a significant dose-related reduction of fetal weight in treated rabbits (Hapke et al., 1971).
7,8-Dihydroxyyangonin. The major urinary metabolite of 7,8-dihydroxyyangonin was p- hydroxy-5,6-dehydro-7,8-dihydrokawain, two minor metabolites were hydroxylated derivatives of this compound. No ring-opened products were detected.
Yangonin. Relatively small amounts of yangonin metabolites were detected in the urine. The three metabolites identified were formed via O-demethylation; the major metabolite was p-hydroxy-5,6-dehydrokawain. No ring-opened products were detected.
Keledjian and coworkers (1988) determined the uptake into mouse brain of dihydrokawain, kawain, desmethoxyyangonin, and yangonin at 5, 15, 30, and 45 minutes following intraperitoneal (ip) administration of 100 mg/kg. After 5 minutes, dihydrokawain and kawain attained maximum concentrations of 64.7 and 29.3 ng/mg wet brain tissue, respectively. Desmethoxyyangonin and yangonin had poorly defined maxima corresponding to concentrations of 10.4 and 1.2 ng/mg wet brain tissue, respectively, and these compounds were more slowly eliminated from brain tissue. When crude kava resin was administered ip at a dose of 120 mg/kg, the concentrations of kawain and yangonin markedly increased (2 and 20 times, respectively) relative to the values measured from their individual injection. Dihydrokawain and desmethoxyyangonin levels remained the same as those established for their individual administration.
Structure Activity Relationships: The kava compounds of greatest pharmacological interest are the substituted a -pyrones or kava pyrones, commonly called kavalactones. Six of the major lactones in kava (yangonin, methysticin, dihydromethysticin, kavain, dihydrokavain, and demethoxyyangonin) were screened for relevant information on carcinogenicity or genotoxicity. No carcinogenicity studies were identified for any of these compounds; data on toxicity, genotoxicity, and metabolism have been incorporated into the preceding text.
- ABC News (1998) The Kava Craze: The Herb that May Relieve Stress and Anxiety. More Information on Kava. 20/20 Airdate, June 22, 1998 [http://www.herbsnmore.com/2020rpt.html]
- Almeida, J.C. & Grimsley, E.W. (1996) Coma from the health food store: interaction between kava and alprazolam. Ann. Intern. Med., 125, 940-941
- Blumenthal, M., ed. (1998) The Complete German Commission E Monographs. Austin, TX, American Bontanical Council, 685 pp
- Botanicals International (1998) Product Specification. Kava-Kava Rhizome Powdered Extract [http://www.botanicalsintl.com/extracts/kavarhiz.html]
- Cawte, J. (1988) Macabre effects of a cult for kava. Med. J. Aust., 148(11), 545-546
- Cawte, J. (1986) Parameters of kava used as a challenge to alcohol. Aust. N.Z. J. Psychiatry, 20(1), 70-76
- Croom, E.M., Jr. & Walker, L. (1995) Botanicals in the pharmacy: New life for old remedies. Drug Top., 139(6), 84-93
- Dentali, S.J. (1997) Herb Safety Review. Kava. Piper methysticum Forster f. (Piperaceae). Boulder, Co, Herb Research Foundation, 29 pp.
- Dialog Information Services (1998) PIERS Imports (US Ports) (File 573) Palo Alto, CA, searched October, 1998 [Accession Nos. 8593852, 10630535, 11061553, 11061648, 12435192, 13076163, 14039088, 14048410, 14372583, 14509429, 14509531, 14509532]
- Duffield, A.M., Jamieson, D.D., Lidgard, R.O., Duffield, P.H. & Bourne, D.J. (1989) Identification of some human urinary metabolites of the intoxicating beverage kava. J. Chromatog., 475, 273-281
- Elixir (1997) Kava Kava Products [http://www.elixirnet.com/newkavaprod.html]
- Femhealth (1998) Femhealth Botanicals pour la Femme [http://www.femhealth.com/femhealth/ kavacalm.html]
- Hapke, H.J., Sterner, W., Heisler, E. & Brauer, H. (1971) Toxicological studies with Kavaform. Farmaco. Ed. Prat., 26(11), 692-720
- Herbal Alternatives (1998) Medicinal Herbs. Kava Kava [http://www.herbalalternatives.com/ kava.htm]
- Herbal Resources (1995) Kavacin. [http://www.herbsinfo.com/pages/pkavacin.htm]
- Hsu, S.Y., Lin, M.H., Lin, L.C. & Chou, C.J. (1994) Toxicologic studies of dihydro-5,6-dehydrokawain and 5,6-dehydrokawain. Planta Med., 60(1), 88-90
- Israili, Z.H. & Smissman, E.E. (1976) Synthesis of kavain, dihydrokavain and analogues. J. Org. Chem., 41(26), 4070-4074
- Jamieson, D.D., Duffield, P.H., Cheng, D. & Duffield, A.M. (1989) Comparison of the central nervous system activity of the aqueous and lipid extract of kava (Piper methysticum). Arch. Int. Pharmacodyn. Ther., 301, 66-80
- Jamieson, D.D. & Duffield, P.H. (1990) Positive interaction of ethanol and kava resin in mice. Clin. Exp. Pharm. Physiol., 17, 509-514 (cited in Dentali, 1997)
- JNC Corporation (1998) Kava-The National Drink of Fiji [http://www.fiji-online.com.fj/trade/jnj]
- Kava King (1998) Kava King [http:www.kavaking.com/order.html]
- Keledjian, J., Duffield, P.H., Jamieson, D.D., Ligard, R.O. & Duffield, A.M. (1988) Uptake into mouse brain of four compounds present in the psychoactive beverage kava. J. Pharm. Sci., 77(12), 1003-1006
- Kinzler, E., Kromer, J. & Lehmann, E. (1991) Effect of a special kava extract in patients with anxiety-, tension-, and excitation states of non-psychotic genesis. Double blind study with placebos over 4 weeks. Arzneim. Forsch., 41(6), 584-588 (Toxline Abstract: 92/029140)
- Lebot, V., Merlin, M. & Lindstrom, L. (1992) Kava: The Pacific Drug, New Haven, CT, Yale University Press, 255 pp.
- Mathews, J.D., Riley, M.D., Fejo, L., Munoz, E., Milns, N.R., Gardner, I.D., Powers, J.R., Ganygulpa, E. & Gununuwawuy, B.J. (1988) Effects of the heavy usage of kava on physical health: summary of a pilot survey in an Aboriginal community. Med. J. Aust., 148(11), 548-555
- McCoy, M., ed. (1997) OPD Chemical Buyers Directory 1998. New York, Schnell Publishing, p. 403
- Mirasol, F. (1998) Botanicals industry posts strong growth in US. Chem. Market Rep., Sept. 28, 4, 12-13
- NLM (1998) RTECS (Registry of Toxic Effects of Chemical Substances), Bethesda, MD, searched October, 1998 [Record Nos. 70908, 70909, 70910, 70911, 70931, 70934]
- Norton, S.A. (1998) Herbal medicines in Hawaii from tradition to convention. Hawaii Med. J., 57(1), 383-386
- Norton, S.A. & Ruze, P. (1994) Kava dermopathy. J. Am. Acad. Dermatol., 31(1), 89-97
- Nutrahance (1998) Feel Good Hance [http://www.nutrahance.com/wellness.htm]
- Peterson, A. (1998) Kava: The Making of an Herbal Superstar. [http://www.betterlivingusa.com/ kava%20paper.htm]
- Rasmussen, A.K., Scheline, R.R. & Solheim, E. (1979) Metabolism of some kava pyrones in the rat. Xenobiotica, 9(1), 1-16
- Reach4Life (1997) Reach4Life Quality Products. Health and Personal Care-Kava Kava. [http:// www.reach4life.com/1239.html]
- Reach4Life (1998) Reach4Life Quality Products. Health and Personal Care. Estroven [http:// www.reach4life.com/10511.html]
- Ripplecreek (1998) Kava Root Extract Plus [http://www.store.ripplecreek.com/rc122.html]
- Ruze, P. (1990) Kava-induced dermopathy: a niacin deficiency? Lancet, 335, 1442-1445
- Schelosky, L., Raffauf, C., Jendroska, K. & Poewe, W. (1995) Kava and dopamine antagonism. J. Neurol. Neurosurg. Psychiatry, 58(5), 639-640
- Shulgin, A.T. (1973) The narcotic pepper - the chemistry and pharmacology of Piper methysticum and related species. Bull. Narc., 25, 59-74
- Singh, Y.N. (1992) Kava: an overview. J. Ethnopharmacol., 37(1), 13-45
- Smith, R.M., Thakrar, H., Arowolo, T.A. & Shafi, A.A. (1984) High-performance liquid chromatography of kava lactones from Piper methysticum. J. Chromatog., 283, 303-308
- Symmetry (1998) Tranquility. [http://www.go-symmetry.com/tranquility.htm]
- Vitanet (1998) Kava Kava - Kava Root Extract Liquid [http://www/viaweb.com/vitanet/ kavakavrootex.html]
- Volz, H.P. & Kieser, M. (1997) Kava-kava extract WS 1490 versus placebo in anxiety disorders - a randomized placebo-controlled 25-week outpatient trial. Pharmacopsychiat., 30(1), 1-5

