
#169 2-TOET
4-ETHYL-5-METHOXY-2-METHYLTHIOAMPHETAMINE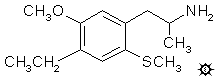
|
| [3D .mol structure] |
To a 21.7 g sample of 2-ethylanisole, well stirred but without solvent, there was added, 1 mL at a time, 21 mL of chlorosulfonic acid. The color progressed from white to yellow, and finally to deep purple, with the evolution of much HCl. The exothermic reaction mixture was allowed to stir until it had returned to room temperature (about 0.5 h). It was then poured over 400 mL cracked ice with good mechanical stirring, which produced a mass of pale pink solids. These were removed by filtration, washed well with H2O, and air dried to give about 27 g of 3-ethyl-4-methoxybenzenesulfonyl chloride as an off-white solid that retained some H2O. A sample recrystallized from cyclohexane had a mp of 44-46 °C. A sample treated with ammonium hydroxide provided white crystals of 3-ethyl-4-methoxybenzenesulfonamide which could be recrystallized from H2O to give tufts of crystals with a mp of 97-98 °C. Anal. (C9H13NO3S) C,H.
In a 2 L round bottomed flask equipped with a mechanical stirrer there was added 200 mL cracked ice, 45 mL of concentrated H2SO4, 26.7 g of still moist 3-ethyl-4-methoxybenzenesulfonyl chloride, and 45 g elemental zinc dust. With external heating, an exothermic reaction set in and the temperature was maintained at reflux conditions for 4 h. After cooling to room temperature, the reaction mixture was filtered and the insolubles washed alternately with H2O and with CH2Cl2. The mother liquors and washings were diluted with sufficient H2O to allow CH2Cl2 to become the lower phase. These phases were separated, and the aqueous phase extracted with 3x100 mL CH2Cl2. The original organic phase and the extracts were pooled, washed with H2O, and the solvent removed to give 15.7 g of a smelly amber oil. This was distilled at 72-84 °C at 0.3 mm/Hg to give 12.1 g of 3-ethyl-4-methoxythiophenol as a water-white oil. The infra-red was perfect (with the SH stretch at 2562, OCH3 at 2837 and 1061, and with fingerprint peaks at 806, 880, 1052, (1061), 1142 and 1179 cm-1). Anal. (C9H12OS) C,H.
To a solution of 11.7 g of 3-ethyl-4-methoxythiophenol and 6.5 mL methyl iodide in 100 mL MeOH there was added, with good stirring and a bit at a time, a solution of 5.5 g 85% KOH in 25 mL hot MeOH. The mixture was held at reflux on the steam bath for 1.5 h, and then stripped of volatiles under vacuum. The residues were added to 400 mL H2O, made strongly basic with 5% NaOH, and extracted with 3x75 mL CH2Cl2. The pooled extracts were back-washed with 1% NaOH, and the solvent removed under vacuum. The 13.2 g residue was distilled giving 2-ethyl-4-(methylthio)anisole as a fraction boiling at 78-85 °C at 0.2 mm/Hg. The weight was 11.6 g for an isolated yield of over 90% of theory. The mp was at about 0 °C. The infra-red showed no SH or other functionality, but an OCH3 at 2832 and 1031, and a fingerprint spectrum with peaks at 808, 970, (1031), 1051, 1144 and 1179 cm-1. Anal. (C10H14OS) C,H.
A solution of 11.2 g 2-ethyl-4-(methylthio)anisole and 9 g dichloro-methyl methyl ether in 200 mL dry CH2Cl2 was treated with 13 g anhydrous aluminum chloride, added a bit at a time. The color progressed from pink to claret to deep claret, with a modest evolution of HCl. Stirring was continued for 1 h, then the reaction was quenched by the cautious addition of 250 mL H2O. The two phase mixture was stirred an additional hour and then separated. The aqueous phase was extracted with 2x100 mL CH2Cl2. The organics were pooled, washed with 5% NaOH, then with saturated brine, and the solvent removed under vacuum. The residue was an amber oil weighing 13.7 g. This was distilled at 0.2 mm/Hg. A first fraction was a yellow oil boiling at 90-100 °C, and weighing 2.9 g. It was a mixture of starting anisole and the desired benzaldehyde. A second fraction, boiling at 100-130 °C was a viscous yellow oil weighing 4.8 g. By TLC it was free of starting anisole, and contained a sizeable quantity of a second benzaldehyde. From this fraction, seed crystal was obtained, and when the oil was dissolved in an equal volume of MeOH, the seed took, producing a yellow solid. This was filtered and air dried, to give 2.2 g of 4-ethyl-5-methoxy-2-(methylthio)benzaldehyde with a mp of 62-63 °C. A small sample from MeOH was almost white, and melted at 61-62 °C. The mixed mp with 4-ethyl-2-methoxy-5-(methylthio)benzaldehyde (57-58 °C) was severely depressed (37-44 °C). A cooled solution of the first fraction of the distillation, in MeOH, provided an additional 1.6 g product, with a mp 59-61 °C. The combined mother liquors gave additional product for an overall weight of 5.3 g. Anal. (C11H14O2S) C,H.
A solution of 1.9 g 4-ethyl-5-methoxy-2-(methylthio)benzaldehyde in 75 mL nitroethane was treated with 0.3 g anhydrous ammonium acetate, and held on the steam bath for 2.5 h. The excess solvent/reagent was removed under vacuum, and the deep orange oil residue was dissolved in 10 mL boiling MeOH. As this cooled, there was the spontaneous generation of crystals. After cooling in an ice bath for a few h, these were removed by filtration, washed with MeOH, and air dried to constant weight. A total of 1.4 g of 1-(4-ethyl-5-methoxy-2-methylthiophenyl)-2-nitropropene was obtained as canary-yellow crystals melting at 83-84 °C which was not improved by recrystallization from MeOH. Anal. (C13H17NO3S) C,H.
To a solution of 1.5 g LAH in 30 mL anhydrous THF that was cooled to 0 °C and stirred under a He atmosphere, there was added, slowly, 1.05 mL freshly prepared 100% H2SO4 (prepared by adding 0.9 g 20% fuming H2SO4 to 1.0 g 96% concentrated H2SO4). This was followed by the addition of a solution of 1.4 g 1-(4-ethyl-5-methoxy-2-methylthiophenyl)-2-nitropropene in 20 mL THF, over the course of 10 min. The color of the nitrostyrene solution was discharged immediately upon addition. With continued stirring, this was allowed to come to room temperature, and then to a gentle reflux for 2 h. After cooling again to room temperature, the excess hydride was destroyed by the addition of IPA. Sufficient 5% NaOH was added to generate the inorganic salts as a loose filterable mass, and these were removed by filtration. The filter cake was well washed with additional IPA, and the combined mother liquors and washes were stripped of solvent under vacuum. The residue was dissolved in 100 mL dilute H2SO4, washed with CH2Cl2, made basic with 5% NaOH, and extracted with 2x75 mL CH2Cl2. Removal of the solvent gave a residue that was distilled at 102-117 °C at 0.15 mm/Hg. The colorless liquid that distilled (0.7 g) was dissolved in 6 mL IPA and neutralized with 11 drops of concentrated HCl. The solids that formed were dissolved by heating the mixture briefly to a boil, and this clear solution was diluted with 20 mL anhydrous Et2O. The white crystals of 4-ethyl-5-methoxy-2-methylthioamphetamine hydrochloride (2-TOET) weighed 0.6 g and had a mp of 164-167 °C. Anal. (C13H22ClNOS) C,H.
DOSAGE: greater than 65 mg.
DURATION: unknown.
QUALITATIVE COMMENTS: (with 50 mg) After about an hour and a half, I found myself a little light-headed. And maybe a feeling of being physically a bit fragile. I ate something, but there was not much joy in eating. And the next day there was some residual fragility, whatever that means. Ahead with caution.
(with 65 mg) During the day this was barely noticeable, but pleasant.
EXTENSIONS AND COMMENTARY: It seems as if the sulfur in the 2-position makes things less interesting, and less potent, than when it is in the 5-position. 2-TOM required twice the dosage of 5-TOM, and here it appears that it could well take a dosage of twice that required for 5-TOET, to get 2-TOET off the ground. There is an understandable reluctance to push on upwards in dosage with a new and unknown compound, when there are feelings of physical discomfort that outweigh the mental effects. There is nothing tangible here. In the complete report of the 50 milligram trial, there is a mention of an inability to effect erection, and this with the light-headedness and disinterest in food, all suggest some involvement with the sympathetic nervous system. And with these subtle effects persisting into the next day, why push higher? Instinct said to leave it alone. So I left it alone.
The 2-carbon analogue, 2C-2-TOET, was made from the same aldehyde intermediate. The appropriate nitrostyrene came smoothly from the aldehyde and nitromethane, and gave glistening pumpkin-orange crystals from methanol, that melted at 93-94 °C. Anal. (C12H15NO3S) C,H. The final phenethylamine hydrochloride salt was prepared from its reduction with aluminum hydride in THF, and was isolated in the usual manner. It was a white crystalline mass that melted at 226-227 °C. It, as with the other 2-carbon analogues of the TOMs and TOETs, remains untasted as of the moment.
| [ |
[Main Index] | [Forward |

